Molecular Adsorbates
We also investigate the properties of various organic molecules on a substrate that is as inert as possible. In the recent past, these have been, in particular, caffeine and theobromine as adsorbates.
Caffeine monolayers on Au(111)
Caffeine is the most commonly used drug and has a wide range of applications in practice. In addition to its effect on the human central nervous system, it is an anticorrosive for copper and can be used to improve the performance and thermal stability of perovskite solar cells.
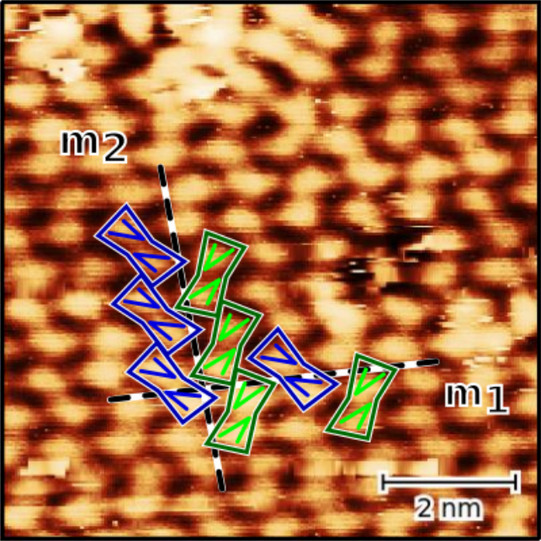
As a crystal, caffeine molecules exhibit polymorphic behavior with an α-phase and a β-phase. Moreover, different orientations have been found in surface-induced phases, one reason being the asymmetric and achiral character of caffeine molecules, which leads to two chiral orientations at the surface. These chiral orientations have an effect on the formation at the surface.
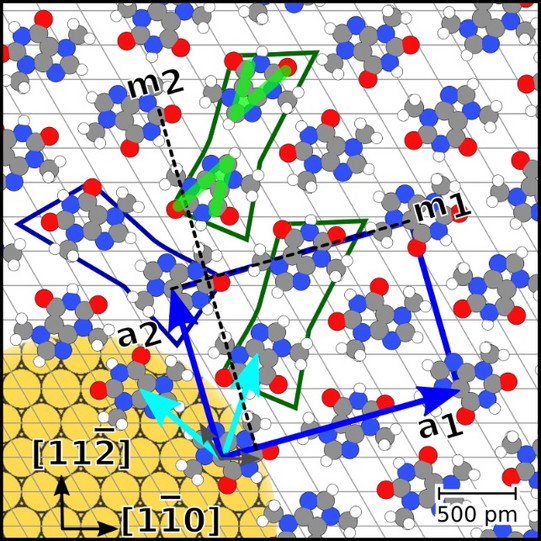
To investigate these effects, the structural formation of caffeine monolayers on an Au(111) surface is analyzed by scanning tunneling microscopy (STM), low energy electron diffraction (LEED), X-ray photoelectron spectroscopy (XPS), and density functional theory (DFT) calculations. The monolayers were prepared by molecular beam epitaxy (MBE) and studied at different temperatures.
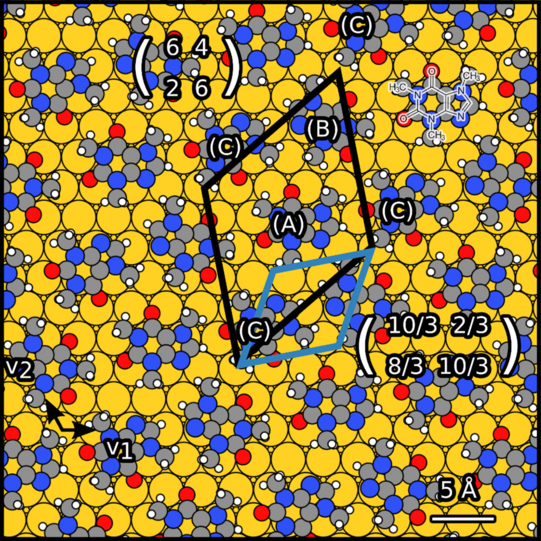
Theobromine monolayers on Au(111) and graphene/SiC(0001).
Theobromine molecules and related compounds such as caffeine and theophylline are important for pharmacology, toxicology and biochemistry. In this field, it is essential to study the formation of chirality. Chirality can arise due to surface adsorption. Therefore, it is necessary to study the chiral adsorption of theobromine on the surface.
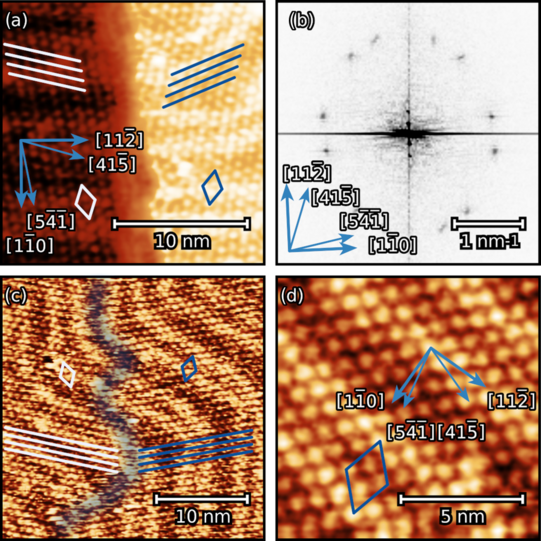
We have studied theobromine monolayers on Au(111) and on graphene with few layers on 6H-SiC(0001). The self-assembly of theobromine was characterized by STM and LEED.
We found that the unit cell of the adsorbate is rectangular and consists of four molecules. Theobromine shows geometrically similar unit cells on both substrates. On Au(111), the molecules are prochiral, with two enantiomers in a zigzag structure explained by slip reflections.